3.4.2.3 Zirconium
Oxide
Zirconium oxide (ZrO2) has gained importance
in the last few years due to its
- high fracture toughness,
- thermal expansion similar to cast iron,
- extremely high bending strength and tensile strength,
- high resistance to wear and to corrosion,
- low thermal conductivity,
- oxygen ion conductivity and
- very good tribological properties (it is very well suited
for slide rings).
Zirconium oxide occurs as monoclinic, tetragonal and cubic
crystal forms. Densely sintered parts can be manufactured
as cubic and/or tetragonal crystal forms. In order to stabilise
these crystal structures, stabilisers such as magnesium oxide
(MgO), calcium oxide (CaO) or yttrium oxide (Y2O3) need to
be added to the ZrO2. Other stabilisers sometimes used are
cerium oxide (CeO2), scandium oxide (Sc2O3) or ytterbium oxide
(Yb2O3).
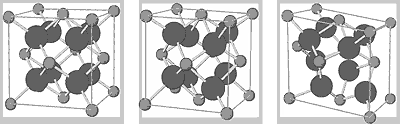
Figure 12: Zirconium oxide: cubic, tetragonal and monoclinic
crystal lattices
light spheres = Zr
dark spheres = O
In fully stabilised zirconium oxide (FSZ – fully
stabilised zirconia) the high-temperature cubic structure
is preserved even after cooling due to the addition of the
other oxides into the crystal structure. The increase in volume,
undesirable for technical applications, does not take place
in FSZ.
Partially stabilised zirconium oxide
(PSZ – partly stabilised zirco-nia) is of great
technical significance. At room temperature, the substance
includes a coarse cubic phase with tetragonal regions. This
state can be retained in a metastable form through appropriate
process control or annealing techniques. This prevents transformation
of the tetragonal phase to the monoclinic phase, and the microstructure
is "pre-stressed"; this is associated with an increase
in strength and toughness.
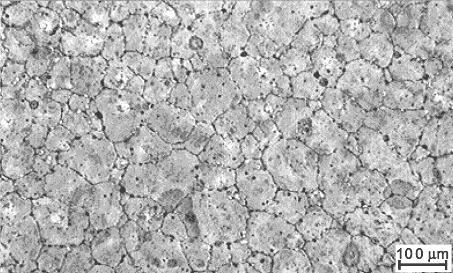
Figure 13: Microstructure of a partially
stabilised zirconium oxide (PSZ)
In polycrystalline tetragonal zirconium oxide (TZP
– tetragonal zirconia polycrystal) the use
of extremely fine initial powders, and the application of
low sintering temperatures, achieves an extremely fine-grained
microstructure. Due to its extremely fine microstructure (grain
size < 100 µm) and the metastable tetragonal structure,
this material is characterised by extraordinary high mechanical
strength, possibly even exceeding 1,500 MPa.
The very finely developed tetragonal crystal
phase in PSZ and in TZP displays a phenomenon unique to high-performance
ceramics: transformation of the tetragonal phase into the
monoclinic phase can be prevented by pressure. When the pressure
is released, e.g. through crack tips or internal tensile stress,
the transformation then occurs. The pressure-controlled increase
in volume involved in the metamorphosis of the crystal phases
closes cracks, slowing or deflecting their growth. This behaviour
is exploited technically, and is known as transformation reinforcement.
In PSZ ceramics, and in particular in TZP ceramics, it leads
to extremely high component strength. Depending on the stabilisation
method, it can be exploited at application temperatures between
600°C and 1,100°C. Zirconium oxide ceramics are therefore
favoured for use in components that are subject to high mechanical
stress.

Figure 14: Microstructure of a polycrystalline
tetragonal zirconium oxide (TZP)
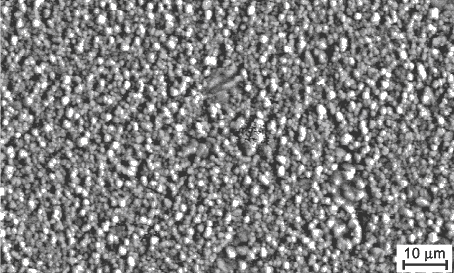
Figure 15: Nanostructure of a polycrystalline
tetragonal zirconium oxide (TZP)
Another property specific to this material is its oxygen ion
conductivity. This phenomenon is used to measure the partial
pressure of oxygen. Zirconium oxide is therefore the basis,
for example, of the "lambda sensors" used to regulate
petrol engine exhausts.
|

